How Retatrutide Works on GLP-1, GIP, and Glucagon Receptors
GLP-1 helps reduce appetite and slows gastric emptying, which promotes satiety. GIP, which was once overlooked, enhances insulin secretion and appears to complement GLP-1 in managing blood glucose levels without amplifying common side effects like nausea. The addition of glucagon receptor activation adds another layer, promoting increased energy expenditure and fat breakdown. Retatrutide, by working on all three, targets metabolic dysfunction from multiple angles at once.
Often referred to as a “triple agonist,” Retatrutide isn’t just another peptide. It works by simultaneously targeting three key metabolic receptors: GLP-1, GIP, and glucagon. Each of these receptors plays a distinct role in regulating appetite, insulin sensitivity, and energy expenditure. When activated together, the combined effect appears to create a powerful synergy in improving metabolic function.
Retatrutide Clinical Trials for Weight Loss and Metabolic Improvements
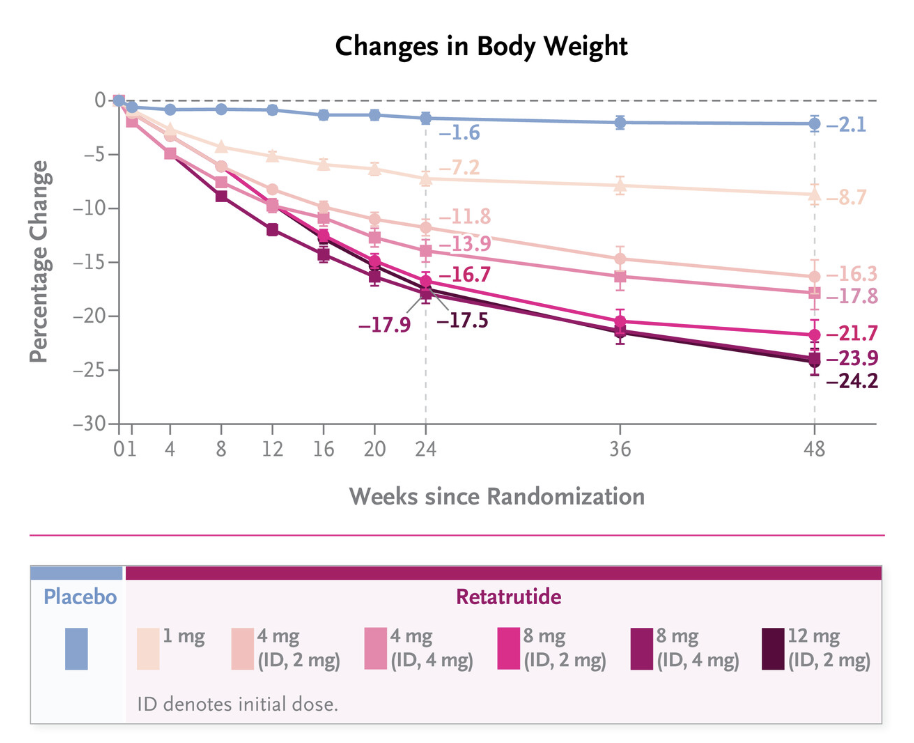
Percentage change in body weight from baseline over 48 weeks in the Retatrutide Phase 2 trial. Data adapted from Jastreboff AM et al., published in The New England Journal of Medicine (2023) – Image Source.
Results from a phase 2 clinical trial captured widespread attention. As the trial approached its halfway point, Retratrutide had a striking weight loss of 17.5% compared to just 1.6% of those who received the placebo. By the end of the trial, the weight loss grew to 24.2% compared to 2.1% of those who did not receive Retatrutide. These results positioned Retatrutide as a potential therapy that could reshape the treatment landscape for obesity and metabolic syndrome.
Beyond weight loss, researchers also track improvements in insulin sensitivity, lipid profiles, and inflammation markers. The therapeutic implications could extend to type 2 diabetes, nonalcoholic fatty liver disease (MASLD), and even chronic inflammatory conditions. In fact, clinical trial findings published in The Lancet suggest that Retatrutide may offer substantial improvements in glycemic control and weight management for patients with type 2 diabetes.
Retatrutide Beyond Weight Loss
The science is compelling, but the story behind Retatrutide is just as interesting. Its development is part of a larger trend in pharmaceutical research, designing molecules that don’t just mimic one hormone but rather orchestrate several pathways at once. Think of it less as a single key turning a lock and more like a conductor guiding an orchestra. This multi-target approach mirrors the complexity of the metabolic disorders it aims to treat.
Retatrutide’s triple receptor action is a leap forward from its predecessors. While GLP-1 and GIP dual agonists like Tirzepatide already showed impressive results, researchers hypothesized that adding a glucagon receptor component might unlock further metabolic benefits. In the carefully balanced framework of Retatrutide, this activation appears to enhance energy expenditure without compromising glycemic control.

This design did not happen overnight. It came after years of trial and error and close observation of how individual receptor pathways influence one another. The ultimate goal was a peptide that could provide comprehensive metabolic control with fewer side effects and broader applications.
What makes Retatrutide particularly promising is not just the scale of weight loss but the quality of the weight lost. Visceral fat reduction, improved fasting insulin levels, and better lipid profiles point towards changes that could impact long-term cardiovascular and metabolic health. In an age where obesity is increasingly viewed as a complex, chronic disease rather than a lifestyle issue, these shifts in biomarkers matter.
Why Retatrutide Signals a Shift in Peptide Therapy
For clinics, the emergence of Retatrutide signals a broader trend; the evolution of multi-pathway peptide therapies. With more complex mechanisms come greater potential benefits but also a need for more nuanced understanding and responsible application. This is not a compound to be approached with a one-size-fits-all mindset. Instead, it invites a more tailored, personal approach to patient care, one that aligns not only with better outcomes but smarter practice growth.
Some forward-thinking clinics are already preparing for what’s to come. Retatrutide’s rapid rise suggests that familiarity now will give clinics a strong foundation for later. Staying current on data, trial outcomes, and emerging peptide education is essential. In a landscape that shifts quickly, being informed is the best way to remain trusted.
Peptides like Retatrutide offer insight into how these advances might shape future treatment protocols. The trajectory of Retatrutide is clear. It represents a new era of precision in metabolic modulation, offering the possibility of treating root causes, not just symptoms.
The information provided in this article is for educational and informational purposes only and is not intended as medical or clinical advice. It should not be used to diagnose, treat, or prescribe for any health condition. Always consult a qualified healthcare professional before making any medical decisions or changes to treatment protocols.
References
Jastreboff AM, Kaplan LM, Frías JP, Wu Q, Du Y, Gurbuz S, Coskun T, Haupt A, Milicevic Z, Hartman ML; Retatrutide Phase 2 Obesity Trial Investigators. Triple-Hormone-Receptor Agonist Retatrutide for Obesity – A Phase 2 Trial. N Engl J Med. 2023 Aug 10;389(6):514-526. doi: 10.1056/NEJMoa2301972. Epub 2023 Jun 26. PMID: 37366315.
Rosenstock J, Frias J, Jastreboff AM, Du Y, Lou J, Gurbuz S, Thomas MK, Hartman ML, Haupt A, Milicevic Z, Coskun T. Retatrutide, a GIP, GLP-1 and glucagon receptor agonist, for people with type 2 diabetes: a randomized, double-blind, placebo and active-controlled, parallel-group, phase 2 trial conducted in the USA. Lancet. 2023 Aug 12;402(10401):529-544. doi: 10.1016/S0140-6736(23)01053-X. Epub 2023 Jun 26. PMID: 37385280.
Want Deeper Insights into How Peptides Intersect with Metabolic Health?
Join our newsletter for early insights into emerging blends, research developments, and strategies shaping the future of peptide therapy.
